Degenerazione maculare senile nel miope
Management of Choroidal Neovascularization in Myopic Macular Degeneration
Past, Present and Future Therapies
Brief Summary
Choroidal neovascularization (CNV) is one of the most important vision-threatening complication secondary
to pathological myopia (PM). In the last two decades, there have been rapid advances in the treatments'
options for myopic CNV. The aim of this work is to give an overview of several modalities of treatment of CNV
in myopes including laser photocoagulation, transpupillary thermotherapy (TTT), radiotherapy, photodynamic
therapy (PDT) with verteporfin, surgical treatments. Based on the new scientific progress achieved in the past
few years, it is possible that patients with myopic macular degeneration complicated from CNV may benefit
from a new ophthalmic frontier of pharmacologic therapy including intravitreal injections of anti-VEGF drugs.
The current status of these novel therapeutic agents is also stressed.
Key Words
Pathological myopia, choroidal neovascularization, natural evolution, laser photocoagulation, photodynamic therapy, anti-angiogenic drugs, intravitreal injection.
Expert commentary
The field of macular degeneration research continues to experience improving therapeutic options in the
manage of choroidal neovascularization.
In myopic macular degeneration, as in age-related macular degeneration, retina specialists
and above all the patients, face the treatment of the disease with new enthusiasm and optimism.
In fact evidence suggests that the angiogenic factor plays to significant role in the development of CNV.
Therefore the possibility to use drugs able to inhibit VEGF opens a window of hope for myopic patients,
often young, with the objective to maintain an visual capability enough for the daily activities as to drive,
to work, to read and to write.
Five year view
The reliability of the new generation of imaging software is going to provide to be a specific tool for
better define the presence of CNV and fluid in the retina also in high myopic patients.
In the next years we will have more effective drugs which will also combined together. We do hope that the
genetic therapy and probably in a longer time term also stem cells or retinal transplantation could be used
in the treatment of degenerative CNV.
Key issues
1. Pathological myopia is frequent and predominant in females
2. PM is often complicated by choroidal neovascularization
3. CNV is prevalently subfoveal and affects people in the forties
4. CNV's natural history is poor with a final Fuchs spot
5. Laser photocoagulation is not considered appropriate for the majority of subfoveal lesions and for
iuxtafoveal CNV
6. Photodynamic therapy is the standard therapy in myopic CNV
7. Intravitreal injection with anti-angiogenic drugs are actually off-label, but will be the best therapeutic
option in the next future
Introduction
Pathological myopia (PM) is one of the leading causes of visual disability in the world from 20 to 50
years of age. (1)
Prevalence rates of PM varies between 2% and 9%, depending on the race and age of the population. The prevalence
of myopia is high especially in parts of East Asia, with rate of 9 - 21%, when compared with 2 - 4% in white
people.
Still unknown is whether this high prevalence reflects primarily genetic factors or a more complex response
to environmental stimulation occurring on a susceptible genetic/ethnic background.(2)
Visual acuity (VA) decreases with severity of myopic refractive error, particularly for myopia higher than 3
diopters.
Myopia was a predictor for visual impairment (odds ratio = 1.26) and for blindness (odds ratio = 1.20) after
five years. It is also present an association of severe myopia with myopic macular degeneration.(3,4)
Pathogenesis
 The pathogenetic mechanisms of myopia are still not completely elucidated, though genetic and enviromental
factors may both play a role.
The pathogenetic mechanisms of myopia are still not completely elucidated, though genetic and enviromental
factors may both play a role.
PM is characterized by excessive axial length (³26 mm) with secondary degenerative changes of the sclera,
choroid, Bruch's membrane, retinal pigment epithelium (RPE), and retina.
These changes are concentrated in the mid-peripheral fundus and in the macular area.
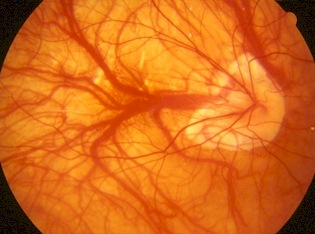
 The most common posterior pole changes include posterior staphyloma, optic nerve crescents, chorioretinal
atrophy, lacquer cracks, and the development of choroidal neovascularization (CNV) in the center of the
macula via vascular endothelial growth factor (VEGF) modulation (Figure 1-2).(5,6)
The most common posterior pole changes include posterior staphyloma, optic nerve crescents, chorioretinal
atrophy, lacquer cracks, and the development of choroidal neovascularization (CNV) in the center of the
macula via vascular endothelial growth factor (VEGF) modulation (Figure 1-2).(5,6)
CNV in pathological myopia
Some other Authors have already reviewed the management of CNV in myopic
macular degeneration and our review is an up-to-date of this disease considering the new therapeutic
options in the last years.(7,8)
CNV is one of the most important vision-threatening complications of PM and occurs in 5% to 10% of
myopic patients.
Myopic CVN is predominant in females (67%) who may reflect estrogen receptor expression in CNV
and external influence of extrogen.(9) The development of CNV is subfoveal in about 58% of cases and
juxtafoveal in 32%.(2) Choroidal neovascularization usually affects young adults in the fourth and fifth
decades of life, and has a poor prognosis with a significant risk of visual deterioration.(10)
Linear breaks in Bruch' s membrane secondary to stretching and degeneration of the choroid take these eyes
at risk for CNV. These breaks often appear clinically as lacquer cracks and are associated with CNV in 82%
of eyes it is unclear, however, what causes the growth of new-vessels through the breaks in Bruch's membrane.(12)
In advanced stage, CNV appears as a Fuchs' spot that is a macular scar with pigment clumping, and sometimes
hyperpigmentation in association with fibrosis and retinal atrophy is present.(13)
Among myopic patients with pre-existing CNV, more than 30% will develop CNV in the fellow eye within 8
years.(11)
Clinical biomicroscopy
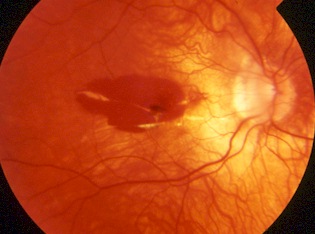
 The slit-lamp binocular macular biomicroscopy shows that CNV appears as a small pigmented or red lump
beneath the retina and often is surrounded by a dark pigment ring with macular hemorrhage (Fig 3).
The slit-lamp binocular macular biomicroscopy shows that CNV appears as a small pigmented or red lump
beneath the retina and often is surrounded by a dark pigment ring with macular hemorrhage (Fig 3).
Typically, myopic CNV is located between the neurosensory retina and the retinal pigment epithelia
l layer.(13)
The sensory retinal elevation associated with this CNV seems to be extremely small, shallow, and difficult
to distinguish clinically because of the minimal accumulation of subretinal fluid and exudation.
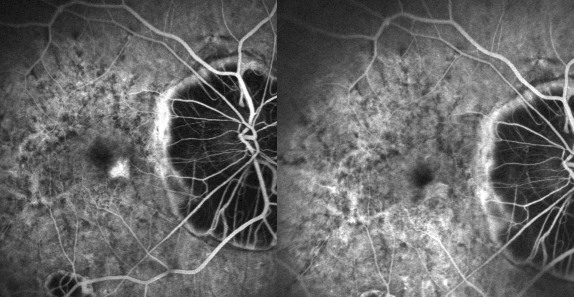
Fluorescein angiography and indocyanine green angiography
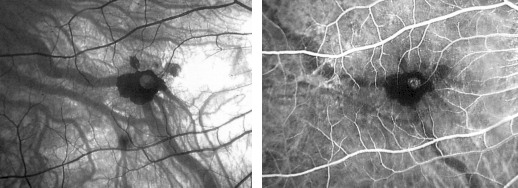
 Fluorescein angiography is the main tool for diagnosing CNV in
myopic eyes. Over 90% of CNV in pathologic myopic eyes demonstrates mild hyperfluorescence confined to the
early phase of the angiogram with minimal leakage beyond the margins of the lesion in the late phase frame
(Fig. 4).
Fluorescein angiography is the main tool for diagnosing CNV in
myopic eyes. Over 90% of CNV in pathologic myopic eyes demonstrates mild hyperfluorescence confined to the
early phase of the angiogram with minimal leakage beyond the margins of the lesion in the late phase frame
(Fig. 4).
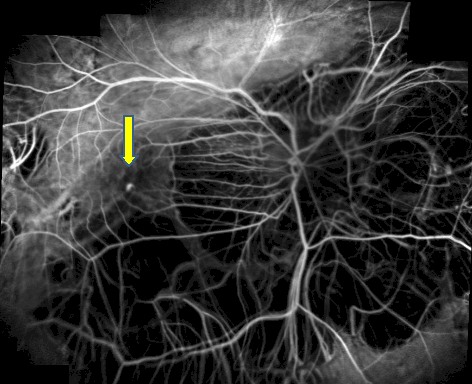 Myopic CNV is usually very small (Fig. 5) and in some cases may be associated with more widespread leakage
and evidence of mild myopic degeneration with relatively unchanged choriocapillaris.
Myopic CNV is usually very small (Fig. 5) and in some cases may be associated with more widespread leakage
and evidence of mild myopic degeneration with relatively unchanged choriocapillaris.
In the clinical practice fluorescein angiography is able to detect the CNV in most of the cases and therefore
indocyanine green angiography (ICG-A) is not useful.
However, when large hemorrhages are present ICG-A may better identify the CNV as a faint hyperfluorescent area
often surrounded by a hypo-pigmented halo. ICG on the contrary allow us to visualize lacquer cracks more
distinctly than fluorescein angiography.(14)
Natural history
 Understanding the natural course of myopic CNV is very important to evaluate and counselling the most
appropriate treatment option in selected cases.
Understanding the natural course of myopic CNV is very important to evaluate and counselling the most
appropriate treatment option in selected cases.
The long-term natural history studies on myopic CNV show that this lesion leads to chronic exudation, subretinal
hemorrhage, and end-stage chorioretinal atrophy developing around the regressed CNV in the central macula.
(16,17)
Previously, several retrospective studies have also investigated the course of untreated CNV in PM, and the
results concluded that the natural history and visual outcome in these eyes is very poor.
Hampton et al 10 found that 43% lost two lines or more of VA and 60% had a VA of 20/200 or less.
Hotchkiss and Fine 13 in a retrospective study also observed a poor visual prognosis and 20 of 27 eyes had a
legal blindness.
Tabandeh et al(18) followed up 22 eyes of 22 patients (50 years of age or older) with myopic CNV and reported
that final VA was 20/200 or less in 73% of the eyes after a mean follow-up period of 49 months.
Secretan et al(19) followed 100 eyes with juxtafoveal CNV for 5 years and all of them developed a subfoveal CNV
with a final VA of 20/160.
In a recent study on the natural evolution of CNV in myopes, Yoshida et al(17) reported a progressive deterioration
of vision that is 20/200 or less in 96.3% of eyes followed for 10 years after the onset of CNV. Several works have
evaluated the prognostic factors in patients with CNV and PM. In a retrospective review of 73 eyes in 63
patients with myopic CNV, patients aged more than 40 years at onset had a poorer initial VA and a significantly
reduced VA during the follow-up.
The patients' age may have influence on the visual result; Hayashi et al(20) and Yoshida et al(21)
have shown that younger patients, smaller CNV, and better initial VA were more likely to have a good visual
prognosis.
Kojima et al(22) reported that areas of chorioretinal atrophy are the most significant cause of poor long-term
visual prognosis, and older age and larger CNV also were determinant factors for the onset of atrophy.
Treatment options for CNV
Choroidal neovascularization in PM has been treated with different approaches that include: laser
photocoagulation, submacular surgery, photodynamic therapy (PDT), transpupillary thermotherapy (TTT), and more
recently intravitreal injections of anti-VEGF (anti-Vascular Endothelial Growth Factor) drugs into the eye,
following the good results achieved by some non randomized clinical study.
However, it should be clear that none of these treatments is a cure for myopic CNV. Each treatment may slow
the rate of vision deterioration or stop further vision loss, but in some eyes the disease and loss of VA may
also progress despite different therapeutical intervention.
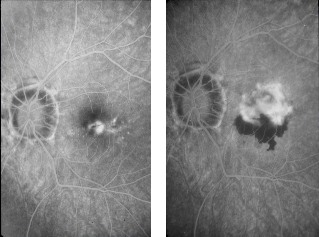
Laser photocoagulation
 Thermal laser photocoagulation has been used for several years. The aim of this treatment was to destroy
the CNV in order to preserve macular function; the success is strongly dependent on its ability to eradicate
the CNV without destroying the fovea.
Thermal laser photocoagulation has been used for several years. The aim of this treatment was to destroy
the CNV in order to preserve macular function; the success is strongly dependent on its ability to eradicate
the CNV without destroying the fovea.
Uncontrolled clinical study have shown that, although conventional laser photocoagulation may stabilize
VA, there is no evidence to suggest benefits in patients with subfoveal CNV.
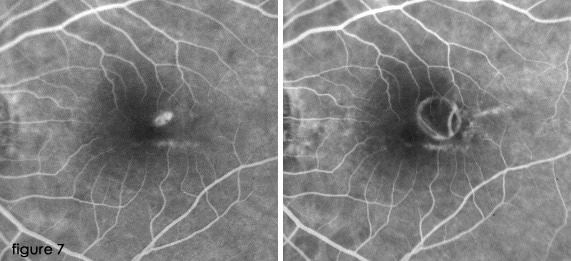
Previous prospective clinical trials have shown that the most widely used treatment for myopic CNV is laser photocoagulation when fluorescein angiography reveals lesions outside the geometric center of the FAZ.
This is the only technique shown to have a significantbenefit in selected patient populations (Fig. 7).
(16,19,24,26)
Generally, laser treatment of CNV in PM is more difficult than the treatment of CNV in age-related macular
degeneration. The causes may be due to hypopigmentation of the fundus that reduces laser light uptake, and
difficulties to focusing CNV at the macular region and evaluating the localization of the foveola in relation
to the neovascular tuft.
Brancato et al(27) have shown that results of treatment performed using different laser sources (green argon,
red krypton, organic dye laser at 590nm) was with no statistically significative differences in the final
outcomes.
However, while initially some statistically significant benefits were reported, they were modest and realized up 2
years after treatment.
In a series of 19 patients treated with laser photocoagualation for extrafoveal CNV associated with myopia,
Jalkh et al(25) found that VA improved in 11% , remained unchanged in 21%, and worsened in 68% of eyes. All
except two eyes showed progressive enlargement of the laser scar.
Secretan et al(19) compared laser photocoagulation versus natural history and concluded that laser treated eyes
had statistically better VA at 2 years but this differences was insignificant after 5 years. Pece et al(24)
prospectively followed 133 eyes with extrafoveal macular CNV treated with direct thermal laser photocoagulation and
reported that laser treatment may be effective in preventing or limiting visual loss in a long-term course.
 Riuz-Moreno et al (26) evaluated visual outcomes in 23 eyes of 21 patients with high myopia (>6.0
diopters and/or axial length >26 mm) with juxtafoveal CNV treated with argon green laser photocoagulation.
Visual acutiy initially improved for a period between 2 and 24 months.
Riuz-Moreno et al (26) evaluated visual outcomes in 23 eyes of 21 patients with high myopia (>6.0
diopters and/or axial length >26 mm) with juxtafoveal CNV treated with argon green laser photocoagulation.
Visual acutiy initially improved for a period between 2 and 24 months.
The improvement fades with time, and is no longer significant after the third year.
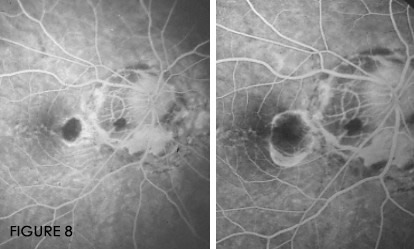
Observational studies suggest that the enlargement of the atrophic laser scar after laser treatment of
non-subfoveal CNV can occur in 92–100% of the treated eyes, the direction of the enlargement following
the morphology of the peripapillary crescent and could be a potentially vision-threatening long-term
complication, even in eyes free of CNVrecurrence (Fig. 8).(19, 24-27)
At the present time, there is no evidence from the randomized, placebo controlled long-term clinical trials to show
that laser photocoagulation is really effective in patients with CNV due to PM on a long term basis.
Laser photocoagulation is therefore not considered appropriate for the majority of subfoveal lesions and for
iuxtafoveal CNV while at this moment we don’t have many data for extrafoveal lesions.
Photodynamic therapy (PDT)
Photodynamic therapy (PDT) with verteporfin of CNV represents an
advancement in the treatment of myopic macular degeneration complicated from CNV. The aim of PDT is to treat
CNV, especially located into the fovea, without the complications of laser photocoagulation.
Originally developed for the treatment of solid tumors, PDT is employed to selectively occlude CNV while
minimizing the degree of injury to the surrounding neurosensory retina. The mechanism of PDT is based on
non-thermal light activation of verteporfin that, administered intravenously, selectively accumulates in the
neovasculature. The result of PDT leads to immediate occlusion of vessels within the CNV.
PDT for subfoveal myopic CNV
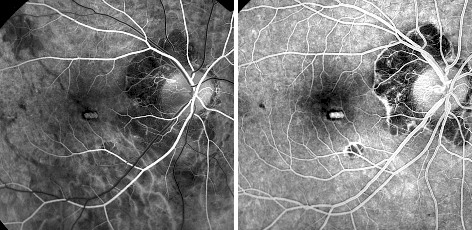
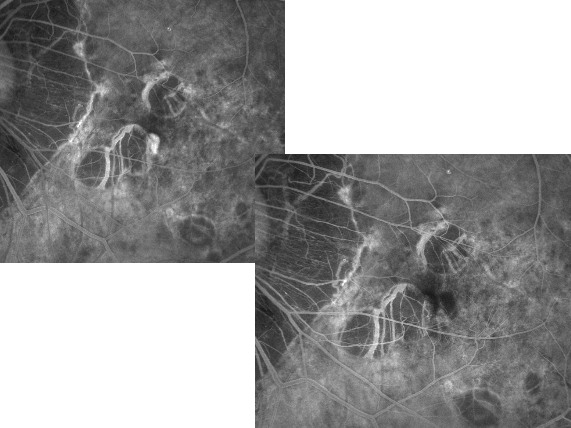 PDT with verteporfin has been demonstrated to improve the visual prognosis of myopic patients with subfoveal
CNV that previously was untreatable with conventional thermal laser (Fig. 9-10).(28,29,30)
PDT with verteporfin has been demonstrated to improve the visual prognosis of myopic patients with subfoveal
CNV that previously was untreatable with conventional thermal laser (Fig. 9-10).(28,29,30)
The Verteporfin in Photodynamic Therapy (VIP) Study Group demonstrated the benefits of PDT at 3 years
post-treatment with a higher final median visual acuity (20/64) compared with untreated patients (20/100).
(31-33)
In summary, 15% of patients had an increase in VA and maintained it during the third year, and 73% maintained
stable vision. Only a few patients lost some vision during the third year and 2% showed a vision increase
during the third year.
 The Verteporfin in Photodynamic Therapy (VIP) Study Group demonstrated the benefits of PDT at 3 years
post-treatment with a higher final median visual acuity (20/64) compared with untreated patients (20/100).
(31-33)
The Verteporfin in Photodynamic Therapy (VIP) Study Group demonstrated the benefits of PDT at 3 years
post-treatment with a higher final median visual acuity (20/64) compared with untreated patients (20/100).
(31-33)
In summary, 15% of patients had an increase in VA and maintained it during the third year, and 73% maintained
stable vision. Only a few patients lost some vision during the third year and 2% showed a vision increase
during the third year.
Pece et al (34) evaluated the safety and effectiveness of PDT with verteporfin in a series of 62 eyes with PM.
The final VA, after a median follow-up of 31 months, improved by 1 or more Snellen lines in 8 patients (13%),
deteriorated in 20 (32%), and remained stable in 34 (55%).
The baseline VA was similar in the various study groups.
The final mean visual acuity in group A (< 55 years old) was 20/80 and significantly (P="0.006)" better than
the 20/138 in group B (>55 years old). The mean final visual acuity in eyes with higher refractive error at
baseline (> –17D) was significantly better (P = 0.014) than in eyes with lower refractive error
(–6 to –10D). The size of the CNV lesion did not affect visual outcomes. Photodynamic therapy preserves
vision in patients with CNV associated with pathologic myopia.
Younger patients ( < 55 years old), and eyes with higher refractive error, appear more likely to benefit from
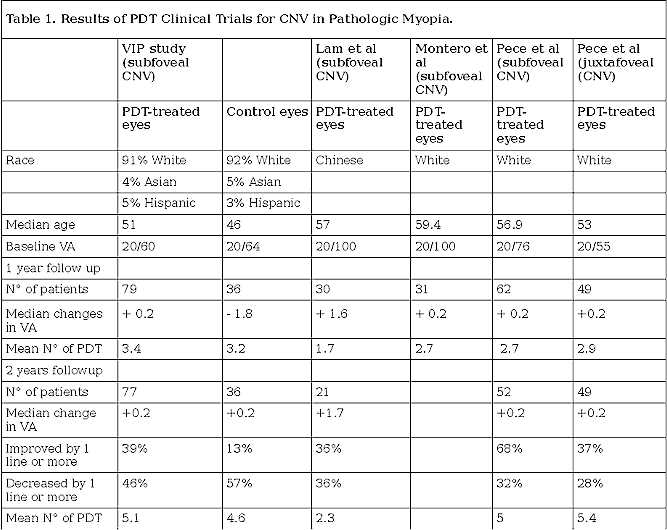
PDT with verteporfin.35 Some results are summarized in table 1.
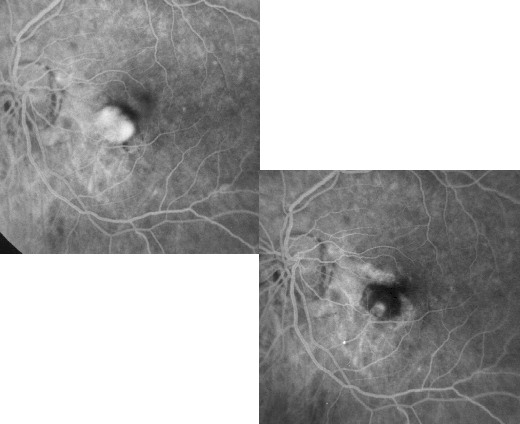
PDT for non-subfoveal myopic CNV
 The major drawback of laser therapy in the treatment of juxtafoveal CNV is the potential for foveolar damage
that could result from an expansion of thermal radiation toward photoreceptor cells into the central fovea or
from an enlargement of laser scar resulting in absolute scotoma with visual disturbances and difficulty in
reading ability.(36)
The major drawback of laser therapy in the treatment of juxtafoveal CNV is the potential for foveolar damage
that could result from an expansion of thermal radiation toward photoreceptor cells into the central fovea or
from an enlargement of laser scar resulting in absolute scotoma with visual disturbances and difficulty in
reading ability.(36)
Verteporfin therapy has been shown to provide significant visual and anatomic benefits for subfoveal myopic
neovascularization.(37) A small case series have also investigated the effect of PDT in the treatment of
juxtafoveal myopic CNV.
Recently, Cohen et al(38) reported a benefit of the treatment on three cases. A small prospective non comparative
trial of 11 eyes with juxtafoveal CNV was performed by Lam et al(39) who found a good response to PDT after 1
year of follow-up. Five eyes (45.4%) improved by three or more lines of vision and 6 eyes (54.5%)
were unchanged.
These authors have similarly concluded that verteporfin therapy is a promising treatment option in treating
juxtafoveal myopic CNV. Another study of Blair et al(40) performed a retrospective evaluation of four patients
with juxtafoveal CNV; in one eye VA improved by 1 lines while in three eyes VA declined by a mean of 3.3 lines
from baseline at 12 month follow-up because the neovascular process continued to growth under the FAZ.
Pece et al(41) have evaluated the effect of photodynamic therapy (PDT) with verteporfin for juxtafoveal CNV in a
series of 48 consecutive patients (49 eyes) with PM followed for 32 months (range, 12 – 56 months). The cohort was
divided into two groups according to age (group A < 55, group B >55 years old), in three subgroups based on
CNV lesion size, and in three categories on the basis of refractive error at baseline.
The results indicated that VA improved by 1 or more Snellen lines in 18 eyes (37%), decreased in 12 eyes (28%),
and remained stable in 19 eyes (39%). Median number of lines gained was 2.14. The average number of lines lost
was 2.38. The final mean VA in group A (mean age: 43.9 years) was 20/50 (logMAR 0.41 SD 0.3) and significantly
better (p = 0.01) than the 20/105 (logMAR 0.72 SD 0.5) in group B (mean age: 67.8 years).
The size of CNV and the magnitude of dioptric error did not influence visual outcomes. The authors concluded
that “PDT is a promising treatment modality for stabilizing and improving vision in 76% of myopic eyes with
juxtafoveal CNV. Younger patients appear to respond more favorably to PDT”.
Surgical treatment of CNV
The surgical treatment of myopic CNV is currently a controversial topic because its is difficult to assess the
exact role of subretinal surgery in the management of patients with subfoveal CNV secondary to PM.
Submacular surgery involves the creation of a retinotomy, usually temporal to the macula, to gain access to the
subretinal space via pars plana vitrectomy. A small case series have been published in the literature.
Maas et al(43) reported that two of four patients who underwent submacular surgery for CNV due to PM had
significant improvement in VA.
Adelberg et al(44) reported that VA had improved by two Snellen lines in two of five myopic eyes with subfoveal
CNV excised by submacular surgery; the remaining three eyes lost no VA following surgery. The Submacular Surgery
Trials (SST), sponsored by National Eye Institute, have determined that sumbacular surgery is not beneficial.
Possible complications after submacular surgery include cataract onset, retinal detachment, recurrences (8 to 57%,
up to 72%17) and RPE defect.(45,46) The major postoperative complication is RPE defect and its expansion
over time. However, the natural history of a myopic CNV also may lead to final scar, that is on average 2.6 times larger than the original CNV.45.
A second type of surgical treatment is the macular translocation: it consists in surgical moving of the central
neurosensory retina or fovea in an eye with recent onset of subfoveal lesion to a new location with presumably healtier RPE and choriocapillaris away from the lesion, where the fovea may be able to recover or maintain its
visual function.(47)
Two different techniques are currently in use to perform macular translocation: a) full macular translocation:
macular translocation with 360-degree or complete circumferential retinotomy; b) limited macular translocation (macular translocation with punctuate retinotomy).
Macular translocation may actually improve visual acuity and reading ability. Full macular translocation has the
best functional outcomes,46 but it also has the highest risk of postoperative complications, such as retinal detachment, macular fold requiring reoperation, macular hole, progression of chorioretinal atrophy.
Limited macular translocation has as major complication insufficient translocation, and therefore enlargement
or recurrence of the CNV, or expansion of the chorioretinal atrophy, involving the new foveal location.
Transpupillary thermotherapy (TTT)
Transpupillary thermotherapy (TTT) with 810nm infrared diode laser
can be another alternative for the management of myopic CNV. TTT differs from standard photocoagulation therapy
in that it uses infrared laser light to apply over a larger area. A low energy, longer exposure diode (810nm)
laser application gently heats up the CNV leading to closure of abnormal neovasculature sparing the overlying
neurosensory retina. Laser energy is applied for 60 seconds.
There are no external drugs used along with this laser treatment as there are in PDT. So there is no risk of
photosensitivity and patient can go back to routine work after the treatment. The goals of TTT treatment are
to seal the leaking vessels and the preservation of the vision. Repeated treatments may be required in recurrent
or persistent CNV.
Few reports on the effect of TTT on myopic CNV have been published in the ophthalmic literature. In a
retrospective analysis of a case series of myopic CNV treated with TTT, Ozdek et al(42) concluded that TTT seems
to stabilize myopic CNVs both clinically and as revealed by angiography, with a significant decrease in the
activity of lesions. Actually TTT considering the new therapeutical options is not any longer used.
New Frontiers for Treating CNV in Pathologic Myopia
Today, new therapeutic options are available for the management of myopic macular degeneration complicated
from CNV. Anti-angiogenic and angiostatic drugs administered intravitreally can represent a new way of
treatment.
Besides, PDT combined with intravitreal triamcinolone acetate, and the anti-Vascular Endothelial Growth Factor
(VEGF) inhibitors are very promising. Since the introduction of intravitreal anti-VEGF, there has been a
gradual reduction in the use of PDT and a new style of patients' management during the post-injection follow-up.
Many patients receiving anti-VEGF agents show visual improvement or stabilization despite long-term leakage
shown on angiography. Herein, we describe the current status of these novel therapeutic agents.
PDT plus triamcinolone acetate
Triancinolone acetate is a corticosteroid with anti-inflammatory properties produced by inhibitory activity
of arachidonic acid formation combined with anti-angiogenic functions. Marticorena et al(48) evaluated visual
acuity changes and safety of combined treatment with photodynamic therapy (PDT) and intravitreal triamcinolone
acetate (IVTA) injection in 12 myopic eyes with CNV in a prospective interventional case series. The combination
of PDT and IVTA may increase the possibility of improving or stabilizing VA in patients with subfoveal myopic CNV. The combination of PDT plus IVTA generally results in the need for fewer PDT retreatment.
Recently Chan et coll(49) treated 22 eyes with a combination of triamcinolone and PDT but the Authors did not
observe a better visual outcome compared with PDT monotherapy, except in selected patients with worse initial
VA or larger myopic CNV.
Anti-vascular endothelial growth factor (anti-VEGF) therapy
The new promising strategies for treating CNV include the use of drugs against the VEGF in particular the
isoform. A which is the most potent promoter of angiogenesis and vascular permeability. This therapy has been
applied for the AMD patients and in the future will be used more diffusely also for myopic CNV .
In general the first ocular anti-VEGF agent is Pegaptanib sodium (OSI/Eyetech Pharmaceutical, New York, USA), an
aptomer designed to target VEGF165, the most representative isoform of VEGF-A in ocular disease. The VISION study
has demonstrated the efficacy of this drug in all CNV subtypes in AMD patients even if patients losing more than
15 letters were still about 40% despite every six weeks injections for two-years study. Literature is very poor
regarding the use of pegaptanib in myopic CNV.
 Bennett et al(55) reported a successful case of pegaptanib for myopic choroidal CNV refractive to laser
photocoagulation, PDT and intravitreal triamcinolone acetonide: visual acuity improved from counting fingers to
20/40 after five, 6-weekly pegaptanib injections.
Bennett et al(55) reported a successful case of pegaptanib for myopic choroidal CNV refractive to laser
photocoagulation, PDT and intravitreal triamcinolone acetonide: visual acuity improved from counting fingers to
20/40 after five, 6-weekly pegaptanib injections.
Bevacizumab (Avastin, Genentech) is an anti-VEGF antibody appoved for the treatment of metastatic colon cancer,
and is used in a off-label way for CNV secondary to AMD, with good short-term safety but uncontrolled series of
patients.
Ruiz-Moreno et al(50) in a prospective, nonrandomized, multicentric, interventional pilot study, described the
anatomical and visual outcome of subfoveal and juxtafoveal CNV in 26 highly myopic eyes treated by intravitreal
bevacizumab.
They concluded that bevacizumab seems to be effective, but further studies are required to verify the efficacy
and usefulness of this therapy compared with established treatments for this condition.
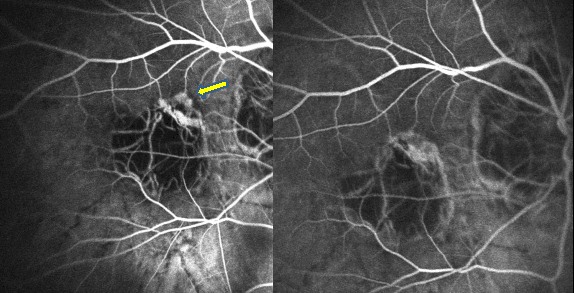
 Chan WM et al(51) also evaluated the safety and efficacy of intravitreal bevacizumab in the treatment of
CNV secondary to pathologic myopia in a prospective, consecutive, nonrandomized, interventional case
series. Twenty-two participants (22 eyes) received an initial course of 3 monthly intravitreal injections
of bevacizumab.
Three additional monthly injections were performed in eyes with persistent CNV leakage after 3 months.
Chan WM et al(51) also evaluated the safety and efficacy of intravitreal bevacizumab in the treatment of
CNV secondary to pathologic myopia in a prospective, consecutive, nonrandomized, interventional case
series. Twenty-two participants (22 eyes) received an initial course of 3 monthly intravitreal injections
of bevacizumab.
Three additional monthly injections were performed in eyes with persistent CNV leakage after 3 months.
Treatment resulted in complete absence of angiographic leakage in 90.9% of eyes at 3 months and 2 (9.1%) eyes
required further treatment up to 6 months.
The 6-month outcomes suggest intravitreal bevacizumab to be a promising treatment method for CNV secondary to
pathologic myopia, resulting in both visual improvement (the mean lines of improvements at 1 and 6 month compared
with baseline were 1.7 and 2.6 lines, respectively) and anatomic improvement.
Other Authors(52-54) reported the effectiveness of bevacizumab in treating this disease, even if the follow up
is short and the number of patients is poor. The Authors stressed that the therapy seems to be safe and
potentially effective. Moreover Yamamoto noted that most of the eye received only 1 injection in a mean
follow up of 153 days.
 Ranibuzumab (Lucentis, Genetech/Roche) is an antibody fragment the binds all active isoform of VEGF-A,
modifying a murine monoclonal antibody to have a fragment for improving VEGF binding. In the Marina study for
minimally classic or occult CNV in AMD patients with monthly injection patients had a stability or improvement
of vision in over 90% of the cases at the end of 24-month study and an improvement of vision in about 30% of the
cases.
Ranibuzumab (Lucentis, Genetech/Roche) is an antibody fragment the binds all active isoform of VEGF-A,
modifying a murine monoclonal antibody to have a fragment for improving VEGF binding. In the Marina study for
minimally classic or occult CNV in AMD patients with monthly injection patients had a stability or improvement
of vision in over 90% of the cases at the end of 24-month study and an improvement of vision in about 30% of the
cases.
Also in ANCHOR trial for predominantly classic CNV secondary to AMD results were even better and therefore
very positive on a 24 –month basis with an improvement of vision up to 40% of patients.
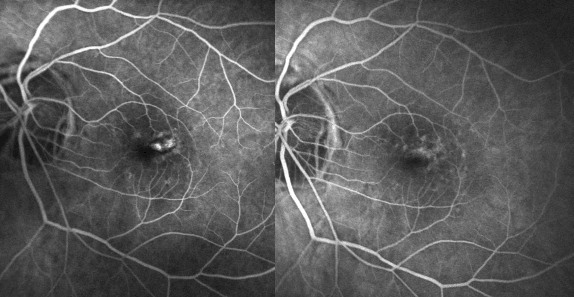
Right now we don’t have any report of ranibizumab in myopic CNV but it seems very promising with good results
and less number of injections (personal experience) (Figure 11-13).
Actually all the drugs described are used as off-label treatments. Problems arise considering their different
cost and returning every month for years is an emotional and psychological burden for not only the patient but
also for the family. Moreover we have to consider the possibility of ocular risks related to the injection
process itself and systemic effects of anti VEGF therapy. About the ocular risks we have to consider that
the complications are decreased dramatically after using better systems of sterility in the operating room,
while for the systemic ones we very poor data and depends on the different drug used. Bevacizumab’s half life
in the systemic circulation is approximately 20 days compared with 12 hour for ranibizumab.
Genentech released data that in SAILOR trial 0,5 dose had a significant risk of stroke compared with
the 0,3 mg dose (1.2% vs 0.3%; P=.02). These data seems to be overexpressed considering the very low incidence
od side effects in the daily clinical experience.
Conclusions
The long-term visual prognosis of CNV in myopic patients is very poor after natural history and also laser
treatment does’nt appear effective on a long term basis. Many prospective clinical studies have shown that PDT
with verteporfin is effective in preserving vision loss of patients with subfoveal and juxtafoveal myopic CNV
that is not amenable with laser photocoagulation. Results can vary depending on patients' age and baseline VA.
In fact, younger patients and patients with higher baseline VA may have a better treatment outcome.
Based on the new scientific progress achieved in the past few years, it is possible that today's patients
with myopic macular degeneration complicated from CNV may profit from a
new ophthalmic frontier of pharmacologic therapy that includes intravitreal injections of anti-VEGF drugs.
However, clinical evaluation in a multicenter, randomized, placebo-controlled trial with longer follow-up is
needed to define more clearly the efficacy and safety of this new modality of treatment.
REFERENCES
1.Ghafour IM, Allan D, Foulds WS. Common causes of blindness and visual handicap in the west of Scotland. Br J Ophthalmol 1983;67:209-213.
2.Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch Ophthalmol
1983;101:405-407.
3.Saw SM, Katz J, Schein OD, Chew SJ, Chan TK. Epidemiology of myopia. Epidemiologic Reviews. 1996; 18(2):
175–187.
4.Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2002 April; 109(4): 704–711.
5.Curtin BJ, Karlin DB. Axial length measurements and fundus changes of the myopic eye. Am J
Ophthalmol 1971;1.42-53.
6.Grossniklauss HE, Green WG. Pathologic findings in pathologic myopia. Retina 1992:12:127-133.
7.Tano Y. Pathologic myopia :where are we now ?Am J Ophthalmol 2002 Nov ; 134 (5) :645-60
8.Chan WM, Ohji M, Lay TY et at. Choroidal neovascularization in pathological myopia : an update in
management. Br J Ophthalmol 2005 Nov ; 89(11) :1522-8.Review
9.Kobayashi K, Mandai M, Suzuma I, et al. Expression of extrogen receptor in the choroidal neovascular
membranes in highly miopic eyes. Retina 2002;22:418-422.
10.Hampton RG, Kohen D, Bird AC, et al. Visual prognosis of disciform degeneration in myopia. Ophthalmology
1983;90:923-926.
11.Avila MP, Weiter JJ, Jalkh AE, et al. Natural history of choroidal neovascularization in degenerative myopia.
Ophthalmology 1984;91:1573-1581.
12.Soubrane G, Coscas G. Choroidal neovascular membranes in degenerative myopia. In: Ryan SJ, ed. Retina.
St Louis, Mo: Mosby-Year Book Inc; 1994:1143-1157.
13.Hotchkiss ML, Fine SL. Pathologic myopia and choroidal neovascularization. Am J Ophthalmol 1981;91:177-183.
14.Brancato R, Trabucchi G, Introini U, et al. Indocyanine green angiography (ICGA) in pathological myopia.
Eur J ophthalmol 1996;6:39-43.
15.Ohno-Matsui K, Morishima N, Ito M, el al. Indocyanine green angiographic findings of lacquer cracks in
pathological myopia. Jpn J Ophthalmol 1998;42:293-299.
16.Yoshida T, Ohno-Matsui K, Ohtake Y, et al. Long-term visual prognosis of choroidal neovascularization in
high myopia. A comparison between age groups. Ophthalmology 2002;109:712-719.
17.Yoshida T, Ohno-Matsui K, Yasuzumi K, et el. Myopic choroidal neovascularization. A 10-year follow-up.
Ophthalmology 2003;110:1297-1305.
18.Tabandeh H, Flynn HW, Scott IU, et al. Visual acuity outcome of patients 50 years of age and older with high
myopia and untreated choroidal neovascularization. Ophthalmology 1999;106:2063-2067.
19.Secretan M, Kuhn D, Soubrane G, Coscas G. Long-term visual outcome of choroidal neovascularization in
pathologic myopia. Natural history and laser treatment. Eur J Ophthalmol 1997;7:307-316.
20.Hayashi K, Ohno-Matsui K, Yoshida T, Kobayashi K, Kojima A, Shimada N, Yasuzumi K, Futagami S, Tokoro T,
Mochizuki M. Characteristics of patients with a favorable natural course of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2005 Jan;243(1):13-9.
21.Yoshida T, Ohno-Matsui K, Yasuzumi K, et el. Characteristic of patients with a favorable natural course of
myopic macular neovascularization. Graefes Arch Clin Exp Ophthalmol 2005;243:13-19.
22.Kojima A, Ohno-Matsui K, Teramukai S, et al. Factors associated with the development of chorioretinal
atrophy around choroidal neovascularisation in pathological myopia. Graefes Arch Clin Exp Ophthalmol
2004;242:114-11.
23.Watzke RC, Packer AJ, Folk JC, et al. Punctate inner choroidopathy. Am J Ophthalmology 1984; 98:572-584.
24.Pece A, Brancato R, Avanza P, et al. Laser photocoagulation of choroidal neovascularization in pathologic
myopia: long-term results. Int Ophthalmol 1995;18:339-344.
25.Jalkh AE, Weiter JJ, Trempe CL, et al. Choroidal neovascularization in degenerative myopia: role of
laser photocoagulation. Ophthalmic Surg 1987;18:721-725.
26.Ruiz-Moreno JM, Montero JA .Long-term visual acuity after argon green laser photocoagulation of juxtafoveal choroidal neovascularization in highly myopic eyes. Eur J Ophthalmol. 2002;12:117-22.
27.Brancato R, Menchini U, Pece A, et al. Dye laser photocoagulation of macular subretinal neovascularization in
pathological myopia. A randomised study of three different wavelengths. Int Ophthalmol 1988;11:235-238.
28.Pérez Oliván S, Torrón Fernández-Blanco C, Ferrer Novella E, et al. Terapia fotodinámica en
neovascularización coroidea. Arch Soc Esp Oftalmol 2004;79:609-616.
29.Lam DSC, Chan W-M, Liu DT, et al. Photodynamic therapy with verteporfin for subfoveal choroidal
neovascularisation of pathologic myopia in Chinese eyes: a prospective series of 1 and 2 year follow-up. Br J
Ophthalmol 2004;88:1315-1319.
30.Ergun E, Heinzl H, Stur M. Prognostic factor influencing visual outcome of photodynamic therapy for subfoveal
choroidal neovascularization in pathologic myopia. Am J Ophthalmol 2004;138:434-438.
31.Verteporfin in photodynamic therapy (VIP) study group. Verteporfin therapy of subfoveal choroidal
neovascularization in pathologic myopia: one-years results of a randomized clinical trial – VIP report n°1.
Ophthalmology 2001;108:841-852.
32.Verteporfin Roundtable 2000 and 2001 Participants, Treatment of Age-Related Macular Degeneration with
Photodynamic Therapy (TAP) Study Group Principal Investigators, and Verteporfin in Photodynamic Therapy (VIP)
Study Group Principal Investigators. Guidelines for using verteporfin (Visudyne®) in photodynamic therapy to
treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina
2002;22:6-18.
33.Verteporfin in photodynamic therapy (VIP) study group. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-years results of a randomized clinical trial – VIP report n°3. Ophthalmology 2003;110:667-673.
34.Pece A, Isola V, Vadalà M, et al. Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization secondary to pathologic myopia. A long term study. Retina Retina. 2006 Sep;26(7):746-51.
35.Montero JA, Ruiz-Moreno JM. Verteporfin photodynamic therapy in highly myopic subfoveal choroidal neovascularization. Br J Ophthalmol 2003;87:173-176.
36.Brancato R, Pece A, Avanza P, Radrizzani E. Photocoagulation scar expansion after laser therapy of choroidal neovascularization in pathologic myopia. Retina 1990;10:239-243.
37.Axer-Siegel R, Ehrlich R, Weinberger D, et al. Photodynamic therapy of subfoveal choroidal neovascularization in high myopia in a clinical setting: visual outcome in relation to age at treatment. Am J Ophthalmol 2004;138:602-607.
38.Cohen SY, Bulik A, Dubois L, Quentel G. Photodynamic therapy for juxtafoveal choroidal neovascularization in myopic eyes. Arch Ophthalmol 2003;136:371-374.
39.Lam DSC, Liu DTL, Fan DSP, et al. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization secondary to pathologic myopia – 1 year result of a prospective series. Eye 2005;19:834-840.
40.Blair MP, Apte RS, Miskala PH, et al. Retrospective case series of juxtafoveal choroidal neovascularization treated with photodynamic therapy with verteporfin. Retina 2004:501-506.
41.Pece A, Vadalà M, Isola V, Matranga D. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization in pathologic myopia: a long-term follow-up study. Am J Ophthalmol. 2007 Mar;143(3):449-54.
42.Ozdek S, Hondur A, Gurelik G, Hasanreisoglu B. Transpupillary thermotherapy for myopic choroidal neovascularization: 1-year follow-up: TTT for myopic CNV. Int Ophthalmol. 2005 Aug-Oct;26(4-5):127-33.
43.Maas S, Deutman AF, Bandhoe F, Aaderkerk AL. Surgical removal of subretinal neovascular membrane. Eur J Ophthalmol 1995;5-48-55.
44.Adelberg DA, Del Priore L, Kaplan H. Surgery for subfoveal membrane in myopia, angioid streaks, and other disorders. Retina 1995;15:198-205.
45.Bottoni F, Perego E, Airaghi P, Cigada M, Ortolina S, Carlevaro G, De Molfetta V.Surgical removal of subfoveal choroidal neovascular membranes in high myopia.Graefes Arch Clin Exp Ophthalmol. 1999 Jul;237(7):573-82.
46.Hamelin N, Glacet-Bernard A, Brindeau C, Mimoun G, Coscas G, Soubrane G. Surgical treatment of subfoveal neovascularization in myopia: macular translocation vs surgical removal. Am J Ophthalmol. 2002 Apr;133(4):530-6.
47.Au Eong KG, Pieramici DJ, Fujii GY, Ng EW, Humayun MS, Maia M, Harlan JB Jr, Schachat AP, Beatty S, Toth CA, Thomas MA, Lewis H, Eckardt C, Tano Y, de Juan E. Macular translocation: unifying concepts, terminology, and classification. Am J Ophthalmol. 2001 Feb;131(2):244-53. Review.
48.Marticorena J, Gomez-Ulla F, Fernandez M, Pazos B, Rodriguez-Cid MJ, Sanchez-Salorio M. Combined photodynamic therapy and intravitreal triamcinolone acetonide for the treatment of myopic subfoveal choroidal neovascularization. Am J Ophthalmol. (2006 Aug;142(2):335-7.
49.Chan WM, Lai TY, Wong AL, Liu DT, Lam DS. Combined photodynamic therapy and
intravitreal triamcinolone injection for the treatment of choroidal neovascularization secondary to pathological myopia: a pilot study. Br J Ophthalmol. 2007;91:174-179.
50.Ruiz-Moreno JM, Gomez-Ulla F, Montero JA, Ares S, Lopez-Lopez F, Rodriguez M, Fernandez M. Intravitreous bevacizumab to treat subfoveal choroidal neovascularization in highly myopic eyes: short-term results. Eye 2007, Dec 7.
51.Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization: six-month results of a prospective pilot study. Ophthalmology 2007 Dec;114(12):2190-6.
52.Yamamoto I, Rogers AH, Reichel E, Yates PA, Duker JS. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularization secondary to pathological myopia. Br J Ophthalmol. 2007;91:157-160.
53.Sakaguchi H, Ikuno Y, Gomi F, et al. Intravitreal injection of bevacizumab for choroidal neovascularization associated with pathological myopia. Br J Ophthalmol. 2007;91:161-165.
54.Hernández-Rojas ML, Quiroz-Mercado H, Dalma-Weiszhausz J, et al. Short-term effects of intravitreal bevacizumab for subfoveal choroidal neovascularization in pathologic myopia. Retina. 2007;27:707-712.
55.Michael D. Bennett, Wendy Yee. Pegaptanib for myopic choroidal neovascularization in a young patient. Graefe’s Arch
Clin Exp Ophthalmol 2007;245:903-905.
